
Georgian College Research Ethics Board
The Georgian College Research Ethics Board (GCREB) serves to approve the ethical integrity of proposed or ongoing research involving human participants that is conducted within, or by members of, Georgian College.
Research [ree-surch]
“An undertaking intended to extend knowledge through a disciplined inquiry or systematic investigation.”
— Tri-Council Policy Statement: Ethical Conduct for Research Involving Humans – TCPS 2 (2018) – Chapter 1: Ethics Framework
Required tutorials
Responsible conduct of college research
All researchers who are conducting college research are advised to read Responsible and Ethical Conduct of College Research, a guide and must complete the related tutorial.
Streamlining the Responsible Conduct of College Research Training Course for non-funded research
- Rather than clicking on each item to find out more as directed by the recording, use the arrow on the right side of the screen to advance the slides.
- Begin on slide 5, unless you want some background on the definition of research and why this tutorial is required.
- Skip Applying for and holding funds on slides 12 and 14.
- Skip Management of grant and award funds on slides 12 and 15.
- Skip Participation in review processes on slides 17 and 18.
- Skip Mismanagement of funds on slides 19 and 30.
- Skip Agency review processes on slides 19 and 32.
- At the end of the tutorial, there are two Tri-Agency policies referenced, and two related Georgian policies:
- AD-015 Research Integrity
- AD-014 Responsible Practice and Ethics Review in Research
Tri-Council Policy Statement (TCPS) 2: CORE-2022 (Course on Research Ethics)
Researchers who wish to conduct research involving humans should complete the online TCPS 2: CORE-2022 (Course on Research Ethics) before they design their protocol. The Tri-Council Policy Statement: Ethical Conduct for Research Involving Humans – TCPS 2 (2022) describes the policies of the Canadian Institutes of Health Research (CIHR), the Natural Sciences and Engineering Research Council of Canada (NSERC), and the Social Sciences and Humanities Research Council of Canada (SSHRC). Researchers must be familiar with the TCPS 2 (2018) before they apply for ethics approval. The TCPS 2: CORE-2022 provides the mechanism for researchers to become acquainted with the TCPS 2, and to provide proof that they have done so.
As of June 30, 2022, the Georgian College Research Ethics Board requires proof of completion of TCPS 2: CORE-2022 for all investigators conducting research at, or under the auspices of, Georgian College. Please submit TCPS 2: CORE-2022 certificates of completion for all investigators with your application. For multi-site studies, you need only submit certificates of completion for the lead principal investigator, any Georgian College investigators, and any investigators who will handle potentially identifiable data about Georgian College site participants.
Navigating the research ethics review process
Before you begin, determine if your project is research involving humans that requires ethics review. See this FAQ.
View this video or download the presentation slides for information on:
- research ethics
- required approvals
- research ethics approval process from start to finish
- common pitfalls and frequently asked questions
Scholarly review
Before applying for research ethics approval, researchers are asked to undergo a scholarly review of their application. Scholarly review is a written critical analysis of the research ethics approval application that examines the appropriateness of the rationale, research questions, hypotheses and research methods. A scholarly reviewer is a person who has demonstrated expertise in either the subject area or research methodology of the research ethics approval application. Here are some examples of checklists used for scholarly review:
- Peer review checklist – Editor resources (taylorandfrancis.com)
- Task Force of Academic Medicine and the GEA–RIME Committee CHECKLIST_OF_REVIEW_CRITERIA
You may also find the GCREB Review Checklist helpful. It can be found under forms.
Forms (save locally before filling out)
Forms for access to resources/recruitment of human participants at Georgian College – An Institutional requirement

Forms for research ethics
Research ethics application forms
- GCREB Application Checklist – Mandatory
- GCREB Application for Research Ethics Approval
- GCREB Change Request
- GCREB Renewal Request
- GCREB Request for Authority to Conduct Course-based Research Ethics Review for course instructors at Georgian College.
GCREB review checklist
This is the form GCREB will use when assessing your application for research ethics approval. You may find it helpful when developing your protocol or seeking scholarly/peer review.
Forms for reports
- GCREB Final Report
- Must be submitted before approval expiry.
- NEW! GCREB Summary of Course-based Research v3
- Excel form for instructors who have been authorized by GCREB to conduct course-based research ethics review.
- Each term, well before research recruitment or data collection begins, complete Part A and submit it to GCREB with the application files for each project.
- After research ends or within two weeks of submitting final grades, complete Part B and submit it with the Final Report forms for each project.
Important notes about forms
- Forms may open in a new tab or may download automatically. Check the top of your browser window or your downloads folder.
- Save your form locally with a unique file name before you begin to work on it.
- If you do not have a digital signature or access to a printer, in lieu of a signature you may insert “By typing my name I am electronically signing this form.” plus your typewritten name, as long as the application is sent from the same email address that you provide on the form. Your Georgian College or other institutional address is preferred.
- For projects occurring at more than one Ontario college, please refer to multi-college research ethics board (expert panel) process and forms.
Policies
Meeting schedule Winter 2025
GCREB deadlines and meeting dates for full reviews
| Application deadline | Review date |
|---|---|
| Tuesday, Apr 29 | Tuesday, May 13 |
| Monday, May 26 | Tuesday, June 10 |
Note: GCREB does not convene in July and August. Fall 2025 schedule will be released late August.
Course-based research ethics review | Information for faculty
The following information may help you to decide if your course requires course-based research ethics approval from GCREB. Research ethics boards (REBs) may delegate ethics review of minimal risk, course-based research to non-REB members, such as faculty.
A. If you answer YES to any of these questions, you need to obtain course-based ethics approval and you must complete the required tutorials:
- Are the assignment objectives connected to providing exposure to research methods?
- Do the assignments in your course require students to practise data collection methods (used in research) with people?
- Are these assignments structured to be minimal risk research activities?
- Note: the determination of minimal risk is from the perspective of the participant. Minimal risk research involves activities or data gathering that is similar to what the participant would experience in their day-to-day life and is unlikely to cause embarrassment, anxiety, be of a personal nature or about a topic that is generally considered private. Any research involving vulnerable populations or Indigenous peoples does not qualify as low risk.
B. If you answer YES to any of these questions, you are not eligible for course-based ethics approval and you MUST submit an individual Application for Research Ethics Approval to GCREB:
- Do your students collect data or engage in the research processes that contribute to your research?
- Will your students all be working on one common research project rather than individual or group projects?
C. The activities below DO NOT require ethics approval:
- Using publicly available information about a person or people to write an essay
- Students talking with a patient or client to learn about their experiences and then using this information to write an essay that will be graded by the teacher
- Skills development activities which are considered standard practice within a profession (e.g. observation, assessment, intervention, evaluation, auditing), provided these activities do not constitute research as defined by the TCPS2. Examples of professional skills development that is not research include:
- students talking with patients or clients (or practising with other students or their teacher) in order to provide a diagnosis, identify appropriate interventions, or give advice
- interviewing someone in a profession about the procedures used at their workplace; this would not be research unless the data were collected in a systematic way to answer a research question such as, “Do Simcoe County businesses follow Human Resources Professionals Association (HRPA) regulations?”
- collecting information from a client to complete their tax return
- collecting information from a client to provide advice or a plan, e.g. a marketing plan
- Research skills using data that was collected anonymously, so long as identifiable information cannot be generated from its use; or using fictional data (e.g. data collected during practice interviews or surveys in which students roleplay rather than divulging personal information)
- For a full list of exemptions, please see the TCPS 2 (2018) – Chapter 2: Scope and Approach.
Next steps
If you have any queries, please contact the REB office at reb@georgiancollege.ca or 705.722.5123.
If you need course-based research ethics approval for your course, be sure to click on requires course-based research ethics approval in Curriculum Information Management (CIM). Additionally, submit your GCREB Request for Authority to Conduct Course-based Research Ethics Review with your course outline/syllabus, related assignment details and TCPS2: CORE-2022 certificate of completion before the submission deadline for GCREB’s monthly meeting. Please see the forms and meeting schedule sections for more information.
Course-based ethics review process
Ensure all investigators complete the required tutorials.
Once you have authority to conduct course-based research ethics review, your students can submit their Requests for Permission for Access to Resources for Research (RPARR), GCREB Application for Research Ethics Approval, any GCREB Change Requests and GCREB Final Report forms to you for each project. As you receive the forms, review them using the GCREB Review Checklist and sign the application form as the principal investigator. If a research project will involve more than minimal risk, you must submit its application and other related forms to GCREB for review.
Before recruitment or data collection begins, submit a GCREB Summary of Course-based Research with copies of all GCREB Applications for Research Ethics Approval for the course to the GCREB.
If your entire class is completing one common project, submit one GCREB Application for Research Ethics Approval for the whole class.
If your students are completing individual or group projects using the same methodology but have different participant groups or documents, submit one GCREB Application for Research Ethics Approval for the whole class, and identify the different investigators’ projects on the Summary of Course-based Research form. Submit any documents particular to each group (e.g. recruitment scripts, informed consent forms, survey or interview questions).
If any changes are made to a research protocol, submit to GCREB a copy of the GCREB Change Request. If any adverse effects occur, report them to GCREB immediately.
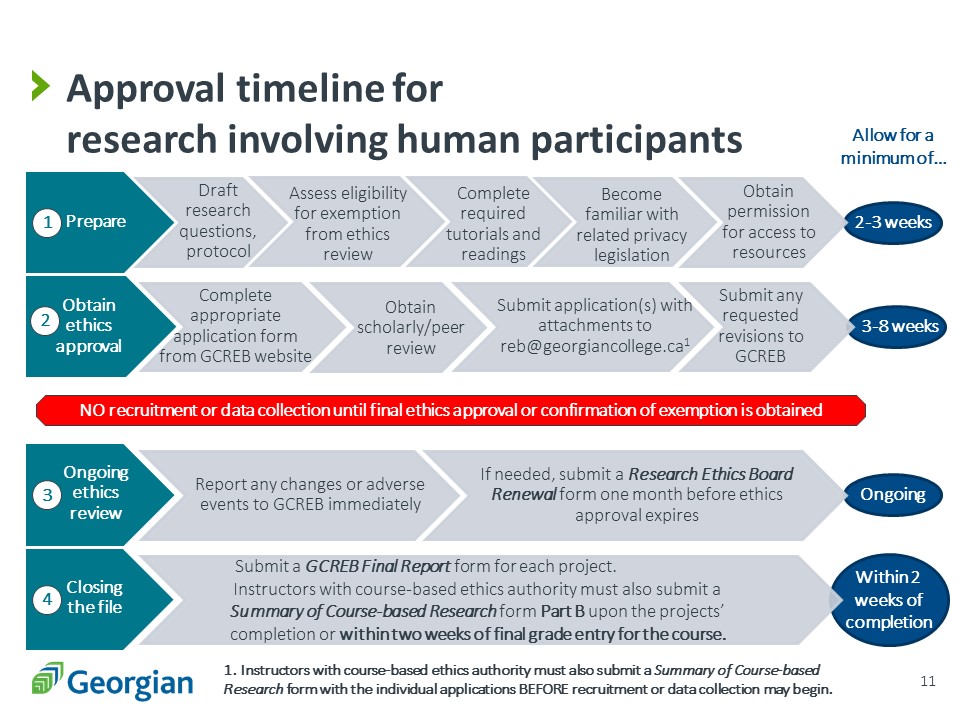
Within two weeks of completion of the research projects or final grade entry, submit to GCREB a fully completed GCREB Summary of Course-based Research with copies of all GCREB Final Report forms and any GCREB Renewal Request forms.
Note: Instead of emailing large files, instructors may wish to save the electronic files for course-based research to a Georgian OneDrive folder and provide access to the GCREB administrative assistant.
Guidelines and frequently asked questions (FAQs)
Yes. Before you get too far into developing your research protocol, you will want to ensure that any resources you are counting on will actually be available to you. If you were to skip this step, you could find that the access or timing you had planned on is unavailable, and you may need to revise your protocol and restart the approval process. Also, because Georgian College receives many requests to involve our students and employees in research, it is important that your planned research does not conflict with other research already occurring at the college, and that there is capacity to add another study. Coordination of research timing can help to boost participation, especially when topics are similar.
Refer to the forms section of this webpage for to access the Request for Permission for Access to Resources for Research (RPARR) form. This form contains a list of the methods often used to recruit research participants at Georgian.
If you’ve had any preliminary discussions regarding access to resources, share this information with the manager who will sign RPARR Section D.
If you don’t know which manager should receive your RPARR, consult the Organizational Charts on the employee portal or contact the Research Ethics Board assistant for guidance. If you are requesting access to both human and non-human resources, submit your RPARR to the manager responsible for access to the most important resource – your research participants – and anyone whose help is essential to your project. Sometimes the topic will dictate who should receive the RPARR. If the resources fall under more than one manager, submit your RPARR to the manager above them. Below are some examples.
- Example 1 – Topic: Academic, e.g. teaching and learning
- If you want to recruit students or employees only from certain academic programs, submit your RPARR to their associate dean.
- If the programs you wish to target fall under more than one associate dean, submit your RPARR to their dean.
- If you wish to recruit participants from programs involving more than one dean or from all academic areas, submit your RPARR to the vice president of academic.
- Example 2 – Topic: Student Success
- If you only want to do research regarding students who use particular service like accessibility or library services, submit your RPARR to the appropriate director of that service.
- If your research is about more than one student service, or if you want to invite all students to participate in a study about student services, submit your RPARR to the executive director of student and learning services.
- Example 3 – Topic: Employment
- If you want to recruit employees from a particular department, submit your RPARR to the manager of that department.
- If you want to invite academic employees from all program areas to participate, submit your RPARR to the vice president of academic.
- If you want to invite employees across the college to participate, submit your RPARR to the vice president of human resources.
International consensus requires that a duly constituted and functioning independent committee review and approve all research projects involving human subjects before the first potential research subject is invited to participate. Independent review reinforces the ethical standards that the researcher and institution strive to meet.
Georgian College of Applied Arts and Technology is eligible to receive funding from two of Canada’s three federal research agencies – the Natural Sciences and Engineering Research Council of Canada (NSERC) and the Social Sciences and Humanities Research Council of Canada (SSHRC). To retain funding eligibility, all research that occurs at or under the auspices of Georgian College must comply with Tri-agencies’ policies, including the Tri-Council Policy Statement: Ethical Conduct for Research Involving Humans – TCPS 2 (2018).
Researchers planning to conduct research involving Georgian employees, students or community members as participants, or research involving humans under the auspices of Georgian or using Georgian resources, must obtain approval from GCREB. The range of research activities requiring review by GCREB includes research that involves living human participants, human biological materials, or human embryos, fetuses, fetal tissue, reproductive materials and stem cells. Please refer to Georgian’s policies on responsible practice and ethics review in research for details.
Some research activities, and research-like activities, are exempt from ethics review. Please refer to Chapter 2 of the Tri-Council Policy Statement: Ethical Conduct for Research Involving Humans – TCPS 2 (2022) for more information.
Still not sure whether your project requires ethics review?
Try the ARECCI Ethics Screening Tool. This is primarily a development/educational tool. It could assist researchers to know if their study requires Research Ethics Board approval. It is sometimes difficult to see the risks involved in your own study. If you find while completing the ARECCI Ethics Screening Tool that the ideas in the questions are unfamiliar or if you feel any hesitation, it is highly recommended that you complete Tri-Council Policy Statement 2 Course on Research Ethics tutorial (TCPS 2 CORE) before returning to the ARECCI Ethics Screening Tool.
Check out these guidelines for differentiating among research, program evaluation and quality improvement from University of Alberta’s Research Ethics Office. This document contains questions in a table format to distinguish research from quality assurance, program evaluation and quality improvement projects.
Researchers should contact the GCREB chair for guidance before deciding not to submit their study. Researchers are advised to save a copy of their ARECCI Ethics Screening Tool results and approved RPARR for discussion with the chair. The chair may also require information such as research questions and data collection tools (e.g. surveys, interview guides) to determine any eligibility for an exemption from ethics review.
Research involving humans being conducted at or under the auspices of Georgian College must have GCREB approval before any recruitment or data collection may begin.
Please see the forms section of this page to download the most recent version of the forms.
If potential subjects can reasonably be expected to regard the probability and magnitude of possible harms implied by participation in the research to be no greater than those encountered by the subject in those aspects of his or her everyday life that relate to the research, then the research can be regarded as within the range of minimal risk.
Minimal risk research:
- draws participants from the general adult population, who are capable of giving free and informed consent, and may not include vulnerable subjects such as children and persons who are who not legally competent to consent;
- does not involve any personal, sensitive or incriminating topics or questions which could place participants at risk;
- does not manipulate behaviour of participants beyond the range of “normal” classroom activity or daily life;
- does not involve physically invasive contact with the research participants;
- does not involve deception;
- does not involve undue or excessive offers of benefit (e.g. an offer of payment in relation to research participation that would exceed the normal range of benefits open to the research participant); and
- may be eligible for delegated ethics review.
- Complete the required tutorials.
- Complete a Request for Permission for Access to Resources for Research (RPARR) and check with any non-Georgian research sites regarding their required approvals.
- Seek out scholarly/peer review of your GCREB Application for Research Ethics Approval or Ontario Community College Multi-site REB Application from someone who has done similar research.
- Submit your completed GCREB Application for Research Ethics Approval or Ontario Community College Multi-site REB Application to GCREB (or to your instructor if applicable).
- Submit additional application components, forms and reports as required by GCREB (or by your instructor if applicable).
- Once you have ethics approval, conduct your research.
- When your research is complete or before ethics approval expires (whichever occurs first), submit a GCREB Final Report or GCREB Renewal Request form to GCREB, or to your instructor, if applicable.
- Your university may require proof that your research will be welcome at Georgian College, and you will want to confirm you have access to the Georgian College resources you will need to conduct your research, so obtain permission for access to resources using the Request for Permission for Access to Resources for Research (RPARR) before applying for ethics approval.
- When you are ready to obtain ethics approval, apply first to the research ethics board at the institution where you are studying.
- Once you have ethics approval at your home institution, apply to the GCREB. Please submit the GCREB Application for Research Ethics Approval or Ontario Community College Multi-site REB Application. Attach your original application along with the signed letter of approval from the other research ethics board. Be sure to include the documents you plan to use at Georgian College.
If the other research ethics board is at an institution eligible to administer grant or award funds from the Canadian Institutes of Health Research (CIHR), the Natural Sciences and Engineering Research Council of Canada (NSERC), or the Social Sciences and Humanities Research Council of Canada (SSHRC), your project may qualify for a delegated approval process.
Please submit the GCREB Application for Research Ethics Approval or Ontario Community College Multi-site REB Application. Attach your original application along with the signed letter of approval from the other research ethics board. Be sure to include the documents you plan to use at Georgian College.
Chapter 3 of the Tri-Council Policy Statement: Ethical Conduct for Research Involving Humans – TCPS 2 (2018) sets out the ethical requirements for obtaining research participants’ “free, informed and ongoing consent”. For the most common data collection methods (e.g. questionnaires, interviews and focus groups), an informed consent form is used to obtain and document consent.
Refer to the GCREB Review Checklist for a list of requirements.
You may find the Health Canada and the Public Health Agency of Canada’s Research Ethics Board Requirements for Informed Consent Documents helpful. You can also find examples of informed consent documents and forms on the University of Waterloo website. Lakehead University’s Template: Letter of Information and Consent Form is available on the Lakehead University website. Ontario Tech University’s template can be found on their ethics resources site.
Remember to remove anything that does not pertain to your project, include the contact information for the research ethics board(s) that will review your study (e.g. GCREB), and to use appropriate letterhead. Only use Georgian College letterhead or branding if you are conducting the research on behalf of Georgian College.
If your research will undergo review by GCREB, you must include the following statement as part of the contact information provided to participants during the informed consent process (preferred) and/or recruitment materials (as appropriate):
- “If you have questions about your rights as a research participant, you may contact GCREB at reb@georgiancollege.ca or 705.722.5123”
Here are some sample demographic questions and categories.
Here are some items to consider before submitting your application:
- Have I addressed all questions in the application?
- Have I included evidence (including references) to back up my rationale and to support my choice of research questions, methodology and tools?
- Have I thoroughly explained my methodology, step by step, including processes that will be used to ensure confidentiality?
- Have I thoroughly explained the experience from the participants’ point of view, including the time commitment, risks and benefits, recruitment process, data collection, debriefing, sharing of results and any incentives that will be offered?
- Have I addressed confidentiality and secure transmission, storage, retention and disposal of data in my research proposal?
- Have I included a copy of all recruiting, interview and debriefing scripts, consent forms, surveys and other supporting documents?
- Have I included proof of completion of the TCPS online tutorial for all investigators?
- Have I signed the application form and initialed where required? Have all investigators signed the Investigator Assurance?
- Have I included the contact information for GCREB on the recruitment materials and/or informed consent documents that I am submitting for ethics approval?
Student researchers:
- If your research is for a course in which the instructor has authority to conduct course-based research ethics review, submit the application and all forms for your project to your instructor.
- Your instructor will sign as the principal investigator.
- If your instructor does not have authority to conduct course-based research ethics review in your course, or if the research is not part of your course work, submit your application and forms directly to GCREB.
- If you are not sure whether your instructor has authority to conduct course-based research ethics review in your course, contact GCREB.
All other researchers:
- Please submit an electronic copy of your application to GCREB via email: reb@georgiancollege.ca.
Submit your complete application at least fourteen working days prior to the next GCREB meeting.
GCREB will often request that a researcher make changes and resubmit their application for reconsideration, so a lead time of at least two months is recommended.
GCREB has regularly scheduled monthly meetings.
Please see the meeting schedule or contact GCREB at 705.418.3359.
No. Applications for research ethics approval are confidential and cannot be shared outside GCREB without the investigator’s permission.
Since approvals comprise most of the business that is conducted at GCREB meetings, they are closed to the public. However, GCREB may invite researchers to provide clarification before conducting their deliberations.
You can expect a response regarding your ethics review in about two to four weeks, depending on the level of review required. When GCREB responds, you may be asked to resubmit your application with changes for additional review, so plan for GCREB approval to take three to eight weeks.
The best way to ensure a quick turnaround is to ensure your application is complete, contains sufficient information to demonstrate compliance with the TCPS 2 (2018) and is submitted at least twelve working days prior to the next GCREB meeting. Applications will not be accepted as complete, and ethics review will not commence, unless accompanied by an approved Request for Permission for Access to Resources for Research (RPARR) and/or documentation of similar administrative approval if the research will use resources of another institution.
You may be asked to address some questions or shortfalls in your application and resubmit it to GCREB for reconsideration.
You also have the right to appeal the decision of GCREB to our Research Ethics Appeal Board (REAB), the Niagara College Research Ethics Board. Such appeals may only be initiated by the research applicant. The research applicant will submit all required materials (the original proposal, the letter of rejection from GCREB and a letter requesting the review) in writing to the chair of the REAB within the allotted time of ten working days. The research applicant requesting the appeal must follow the procedures outlined in Georgian’s procedures on Responsible Practice and Ethics Review in Research and Niagara College’s practice on Research Involving Human Subjects. The decision of the REAB is final.
Please submit a GCREB Change Request if you wish to make any changes to your approved protocol.
For studies with approval at multiple Ontario colleges, you may want to use the multi-college change request form.
Yes. On or before the date your ethics approval expires, please submit a GCREB Final Report and a copy of your final research report to GCREB.
If you decide not to complete your research, submit a GCREB Final Report to GCREB to close the file.
If your research project will go on longer than one year, you must submit a GCREB Renewal Request at least two weeks before the date your ethics approval expires.
For multi-college forms please visit the Multi-College Research Ethics Board website.
Please address any questions to Avinash Thadani, PhD, Chair, Georgian College Research Ethics Board at 249.388.1291.
You may also contact the REB office at reb@georgiancollege.ca or 705.722.5123.
GCREB members
Avinash Thadani, PhD (Chair)
Professor, Collaborative Bachelor of Science in Nursing (BScN) program, Georgian College
Isabelle Deschamps, PhD
Professor, Honours Bachelor of Counselling Psychology degree, Georgian College
Amanda Duncan, MA
Academic Project Manager, Georgian College
Shane Ellis, LL.M., J.D./LL.B., B.A.
Coordinator, Paralegal program, and Professor, Business and Management, Georgian College
Alison Kossowski, PhD, LLB, MSEd, BSc (Hons)
Lawyer
Community member
Brenda J. Marshall, PhD
Professor, Honours Bachelor of Business Administration (Management and Leadership) degree, Georgian College
2024/25
Barbara McNeice-Stallard, MA
Institutional Research, Health care
Community member
Heidi Parekh, BSc
Psychology Community member
Jenna Peters, BA
Research Analyst, Institutional Research, Georgian College
Hanna Shrolyk, PhD Ed
Educational Research, Academic Integrity, Georgian College
David Wegman, PhD
Professor, Pre-health Sciences Pathway to Advanced Diplomas and Degrees program, Georgian College
Amanda Williamson
Honours Bachelor of Nursing program
Georgian College, Student member
Become a GCREB member
GCREB seeks employees and students of Georgian, and volunteer community members with no current affiliation to the college, to serve as GCREB members.
Benefits
- Engaging conversation
- Personal and professional development
- Opportunity to build experience
- Opportunity to learn about ethical conduct of research involving human participants
- Contributing to the ethical integrity of research involving Georgian researchers and participants
Criteria for member selection
- Respect for ethics and responsible practice in research
- Willingness to learn and follow college policies and guidelines
- Willingness to attend meetings and pursue learning opportunities
- Willingness to read learning materials related to ethics and responsible practice in research
- Ability to work with a team
- Ability to respond quickly to requests for review
- Demonstrated commitment to fostering ethical research practices
- Previous experience conducting research involving human participants or as a research participant
- Completion of required training
- Ability to maintain confidentiality
- Good time management
Additional details
- GCREB members are expected to be versed in the procedures and language of research policy. All GCREB members must complete the Tri-Council Policy Statement 2 Course on Research Ethics tutorial (TCPS 2 CORE) in a timely manner, and complete additional research ethics readings before participating in ethics review decisions.
- Meetings are scheduled monthly from late August to early September through June, and are typically held at the Barrie or Orillia campus. Web conferencing is available for those who cannot attend on campus.
- In between meetings, members take turns conducting delegated review of applications.
- Members are asked to assume a two-year term that may be renewable for a maximum of four consecutive years.
- To be considered for GCREB membership, please submit your resumé or CV, and a description of your research experience, to reb@georgiancollege.ca.
- If you require more information, please contact the REB office at reb@georgiancollege.ca.
Contact us
Georgian College Research Ethics Board
Avinash Thadani, PhD
Chair, GCREB
Phone: 249.388.1291
Email: Email Avinash
Prem Batten, RDH, BSc (Hons)
REB Assistant
Phone: 705.722.5123
Email: reb@georgiancollege.ca

